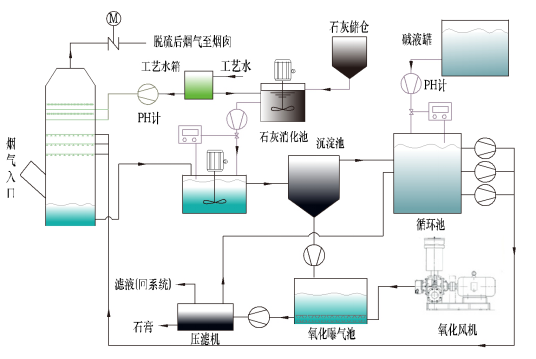
Brief introduction of double alkali desulphurization
The double alkali method is a sodium base desulphurization mechanism for internal desulfurization. Because the sodium base desulfurizer is alkaline and absorbs sulfur dioxide, the solubility of the reaction product is large, and it will not cause supersaturated crystallization and cause the problem of scaling and clogging. On the other hand, the desulphurization product is regenerated with calcium hydroxide in the regeneration pool and regenerated sodium base desulfurization. The agent is then recycled back to the desulphurizing tower.

Working principle of double alkali desulphurization
(1) the dual Alkali FGD technology uses sodium hydroxide or sodium carbonate solution as the starting desulphurizer. The preparation of sodium hydroxide or sodium carbonate solution directly into the desulphurization tower is used to remove the SO2 in the flue gas to achieve the purpose of flue gas desulfurization. Then the desulphurization product is regenerated by the desulfurizer regenerating pool and regenerated by sodium sulfite or sodium hydroxide. Recycle in the desulfurization tower.

The desulphurization process mainly includes 5 parts:
1) the preparation and supplement of the absorbents;
2) the absorbent slurry spray;
3) the mist droplets in the tower are in contact with the flue gas.
4) regenerating pool slurry to reduce sodium base alkali;
5) gypsum dehydration treatment.
(2) the chemical reaction of double alkali desulphurization:
①Absorption reaction
SO2+H2O=H2SO3
SO3+H2O=H2SO4
The SO2 in the main tower is absorbed by sodium alkali solution.
2NaOH+SO2=Na2SO3+H2O
There are still some NaOH in the absorption solution, so sodium sulfite is also formed during the absorption process.
Na2SO3+SO2+H2O2NaHSO3
The SO2 and SO3 in flue gas are absorbed by sodium carbonate solution in the main tower.
E. Na2CO3+H2SO3=Na2SO3+H2O+CO2
F. Na2CO3+H2SO4=Na2SO4+H2O+CO2
② Regenerative reaction
The absorption liquid flows into the reaction tank to react with the added lime slurry.
G.2NaHSO3+Ca(OH)2=Na2SO3+CaSO3·?H2O↓+?H2O
H.Na2SO3+Ca(OH)2+?H2O=2NaOH+CaSO3·?H2O↓
After the regenerated slurry is precipitated by calcium salt, the Na2SO3 clear liquid is sent back to the absorber for recycling.
③side reaction
The main side reaction of the absorption process is the oxidation reaction:
I. Na2SO3+?O2Na2SO4
Therefore, during regeneration, Na2SO4 has the following reactions:
J. Na2SO4+Ca(OH)2+2H2O→2NaOH+CaSO4·2H2O↓
But in fact, because there is a considerable amount of SO or OH in the solution, the concentration of Ca is very low, so it is necessary to precipitate CaSO4, OH less than 0.14M at the time of regeneration, and to have enough SO concentration, such as OH concentration 0.1M, SO concentration for 0.5M, will produce CaSO4 precipitation.。

雙堿法脫硫工藝流程圖
Transportation out of the factory:
 |  |
 |  |